$937.00
Assessment of the risk to develop HIT/HITT, in patients treated with heparins (Unfractionated or LMWH): presence of antibodies is a risk indicator for development of HIT/HITT.
Product Description
Kit of 96 tests
Assessment of the risk to develop HIT/HITT, in patients treated with heparins (Unfractionated or LMWH): presence of antibodies is a risk indicator for development of HIT/HITT.
Product Characteristics
Total Assay Time: 2 hr 15 min
Cut Off: A450 ≥ 0.50
Dynamic Range: A450 up to 3.0
Intra-Assay CV: 4 to 6%
Inter-Assay CV: 5 to 8%
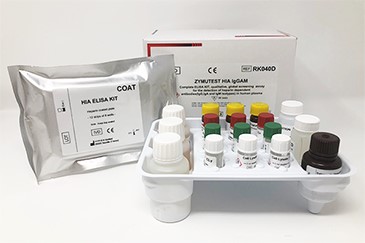
Product Components
Micro ELISA Plate (12 strips of 8 wells)
2x HIA Sample Diluent.
3x HIA IgGAM Positive Control.
3x Negative Control.
3x Platelet Lysate.
3x Anti-IgG (Fcγ)-IgM (µ)-IgA(α)-HRP IC
1x Conjugate Diluent.
1x Wash Solution.
1x TMB.
1 x 0.45M Sulfuric Acid.
![]() Video of Product Components(Video can be viewed with Flash Plugin)
Video of Product Components(Video can be viewed with Flash Plugin)
Product Application
Assessment of the risk to develop HIT/HITT, in patients treated with heparins (Unfractionated or LMWH): presence of antibodies is a risk indicator for development of HIT/HITT.
Clinical suspicion of HIT during heparin therapy.
Related Products