$515.00
Find a wealth of information on this product and order the kit of 32 tests online, here.
PRODUCT DESCRIPTION
In EU: This Product is CE Marked. Health Canada Licensed. Enzyme Immuno-assay designed for measuring heparin-dependent antibodies of the IgG isotype, in human plasma or serum, or in any biological fluid where these antibodies must be measured. This assay measures only the IgG isotype, reported as the most associated with the clinical complications of heparin dependent antibodies (HIT/HITT), allowing confirmation of the diagnosis of HIT/HITT or its clinical suspicion. However, some cases associated with only IgM and/or IgA isotypes can be missed. This kit allows individual test as it offers a packaging for 4 individual 8 well strips along with controls.
Find a wealth of information on this product and order the kit of 32 tests online, here.
PRODUCT CHARACTERISTICS
Total Assay Time: 2 hr 15 min
Cut Off: A450 > 0.30
Dynamic Range: A450 up to 3.0
Intra-Assay CV: 4 to 6%
Inter-Assay CV: 5 to 8%
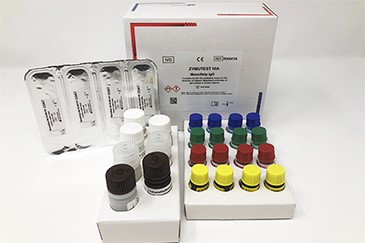
PRODUCT COMPONENTS
Micro ELISA Plate (4 strips of 8 wells)
2x HIA Sample Diluent
4x HIA IgG Positive Control
4x Negative Control
4x Platelet Lysate
4x Anti-IgG (Fcγ)-HRP IC
1x Conjugate Diluent
2x Wash Solution
1x TMB
1 x 0.45M Sulfuric Acid
![]() Video of Product Components
Video of Product Components
PRODUCT APPLICATION
Individual test.
Clinical suspicion of HIT during heparin therapy.
Heparin-dependent antibodies of the IgG isotype are those strongly associated with the clinical diagnosis of HIT. The ZYMUTEST™ HIA MonoStrip IgG assay offers then a better specificity for the clinical complication of HIT, but it has less sensitivity as cases associated with only IgM and/or IgA isotypes are missed.
Related Products






